TG Therapeutics Announces FDA Accelerated Approval of UKONIQ™ (umbralisib)

TG Therapeutics, Inc. (NASDAQ: TGTX), today announced the U.S. Food and Drug Administration (FDA) has approved UKONIQ™ (umbralisib), for the treatment of adult patients with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20 based regimen and adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least three prior lines of systemic therapy.
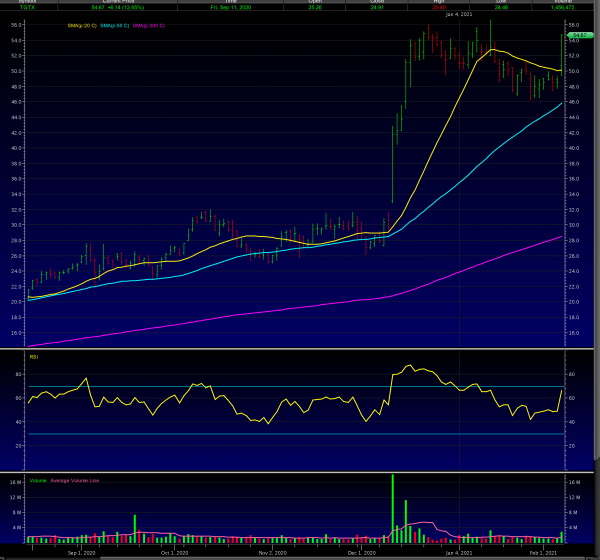
The stock is looking to break previous highs on strong volume.
If so, mid-60’s is next short-term target
UKONIQ is the first and only, oral, once daily, inhibitor of phosphoinositide 3 kinase (PI3K) delta and casein kinase 1 (CK1) epsilon. Accelerated approval was granted for these indications based on overall response rate (ORR) data from the Phase 2 UNITY-NHL Trial (NCT02793583). Continued approval for these indications may be contingent upon verification and description of clinical benefit in a confirmatory trial. This application was granted priority review for the MZL indication. In addition, UKONIQ was granted Breakthrough Therapy Designation (BTD) for the treatment of MZL and orphan drug designation (ODD) for the treatment of MZL and FL.
ABOUT TG THERAPEUTICS, INC.
TG Therapeutics is a fully-integrated, commercial stage biopharmaceutical company focused on the acquisition, development and commercialization of novel treatments for B-cell malignancies and autoimmune diseases. In addition to an active research pipeline including five investigational medicines across these therapeutic areas, TG has received accelerated approval from the U.S. FDA for UKONIQTM (umbralisib), for the treatment of adult patients with relapsed/refractory marginal zone lymphoma who have received at least one prior anti-CD20-based regimen and relapsed/refractory follicular lymphoma who have received at least three prior lines of systemic therapies. Currently, the Company has two programs in Phase 3 development for the treatment of patients with relapsing forms of multiple sclerosis (RMS) and patients with chronic lymphocytic leukemia (CLL) and several investigational medicines in Phase 1 clinical development.
The opinions expressed in this article are the writer’s own and do not constitute financial advice in any way whatsoever. None of the content or data published by Prism MarketView constitutes an investment recommendation, nor should it be relied upon for investment activities. Prism MarketView strongly recommends that you perform your own independent research and/or speak with a qualified investment professional before making any financial decisions. See our full disclaimer at https://demo4.limegoat.com/








